COVID-19 vaccine available for kids under 5 years
The Commonwealth Healthcare Corp. and the Governor’s COVID-19 Task Force, with guidance from the U.S. Department of Health and Human Services, the Centers for Disease Control and Prevention, and the Food and Drug Administration, will be offering COVID-19 vaccines to children under 5 years old (ages 6 months to 4 years old).
COVID-19 booster shots are also available to children aged 5-11 at least five months after their primary series. Eligible individuals can obtain a COVID-19 primary or booster shot by calling the CHCC Immunization Clinic at (670) 236-8745.
COVID-19 vaccines are also available at the following: Medical Associates of the Pacific, Kagman Isla Community Health, Marianas Medical Center, as well as Tinian Health Center, Rota Health Center, Family Care Clinic, Children’s Clinic, and Women’s Clinic. Contact each clinic for more information.
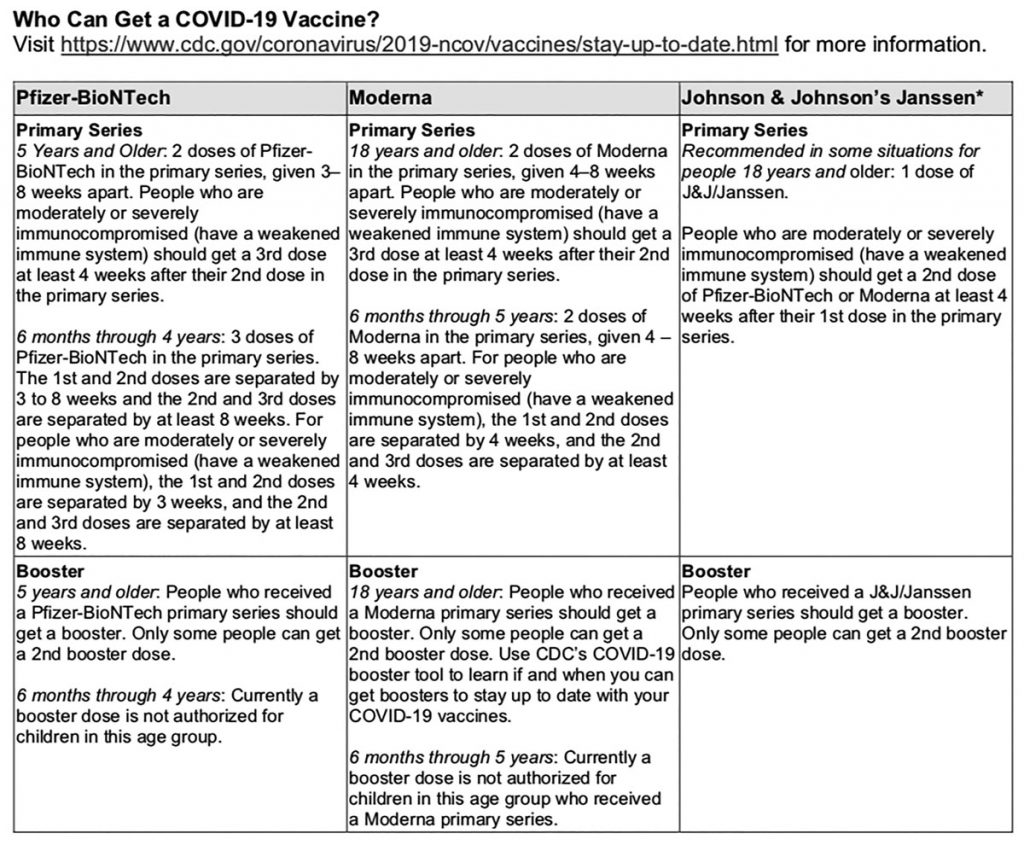
For more information on receiving a booster shot, visit https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html.
Young children are at risk for contracting COVID-19, and some can get seriously ill. Even if a child does not get severely ill, they could face long-term health consequences or pass the virus to others. The best way to protect children against COVID-19—in daycare, school, sports, and with their friends—is by getting them vaccinated.
The COVID-19 vaccines for young children are safe and effective. Like other pediatric vaccines, these vaccines were thoroughly tested and then reviewed by the FDA and CDC before these agencies authorized and recommended them for wide use. The vaccines will be available at no cost and regardless of citizenship or insurance status.
According to the CDC, emerging evidence indicates that people can get added protection by getting vaccinated after having been infected with the virus that causes COVID-19. Even if a child has had COVID-19, they should still get vaccinated. For children who have been infected with COVID-19, their next dose can be delayed 3 months from when symptoms started or, if they did not have symptoms, when they received a positive test. This possible delay can happen with a primary dose or a booster dose.
The COVID-19 vaccines approved and authorized in the U.S. continue to protect people from getting seriously ill, being hospitalized, and even dying—especially people who have received a booster.
Adverse reactions reported after getting booster shots were similar to the primary series/shot. Fatigue and pain at the injection site were the most commonly reported side effects, and overall, most side effects were mild to moderate. However, as with the 2-shot primary series, serious side effects are rare but may occur. (PR)