DUE TO THE PRESENCE OF BENZENE
Bayer voluntarily recalls specific Lotrimin and Tinactin spray products
The Department of Public Health and Social Services in Guam, in coordination with the U.S. Food and Drug Administration, would like to inform the public of a voluntary recall by Bayer of specific Lotrimin and Tinactin spray products due to the presence of benzene.
Bayer is voluntarily recalling all unexpired Lotrimin AF and Tinactin spray products with lot numbers beginning with TN, CV or NAA, distributed between September 2018 to September 2021, due to the presence of benzene in some samples of the products. Benzene is not an ingredient in any of Bayer Consumer Health products. It is important to note that Bayer’s decision to voluntarily recall these products is a precautionary measure and that the levels detected are not expected to cause adverse health consequences in consumers.
As per the USFDA, benzene is classified as a human carcinogen. Exposure to benzene can occur by inhalation, orally, and through the skin. Depending on duration and level of exposure, it can result in cancers including leukemia, and blood cancer of the bone marrow and blood disorders which can be life-threatening. Benzene is found in the environment from natural sources and human activity. Humans around the world are exposed to the substance from multiple sources and pathways, including inhalation, through the skin, and orally. To date, Bayer has no known reports of adverse events related to this recall.
The affected Lotrimin and Tinactin spray products are over the counter antifungal products, sold individually or in combo-packs, packaged in aerosol spray cans. The impacted products are:
– Lotrimin Anti-Fungal (AF) Athlete’s Foot Powder Spray
– Lotrimin Anti-Fungal Jock Itch (AFJI) Athlete’s Foot Powder Spray
– Lotrimin Anti-Fungal (AF) Athlete’s Foot Deodorant Powder Spray
– Lotrimin AF Athlete’s Foot Liquid Spray
– Lotrimin AF Athlete’s Foot Daily Prevention Deodorant Powder Spray
– Tinactin Jock Itch (J1) Powder Spray
– Tinactin Athlete’s Foot Deodorant Powder Spray • Tinactin Athlete’s Foot Powder Spray
– Tinactin Athlete’s Foot Liquid Spray
There are no issues of concern with Lotrimin/Tinactin creams, including Lotrimin Ultra, or any other Bayer products.
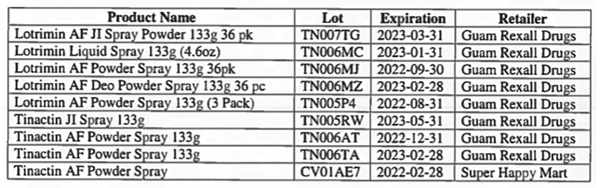
The Division of Environmental Health of DPHSS was notified by two retailers (Guam Rexall Drugs, located in Tamuning, and Super Happy Mart, located in Barrigada), that they had the affected products on hand. Guam Rexall Drugs removed 51 of the affected products, while Super Happy Mart removed one affected product from store shelves.
To date, DEH has determined that the affected Lotrimin AF and Tinactin aerosol spray cans were sold at the aforementioned retailers:
DEH continues to conduct its recall effectiveness check activities and will continue to update the public as more information is obtained.
The Department has not received any local report of injuries or illnesses associated with the use of these recalled commodities. Anyone concerned about a reaction should contact their healthcare provider. Consumers who have purchased the items listed above are urged not to use the affected products. (PR)